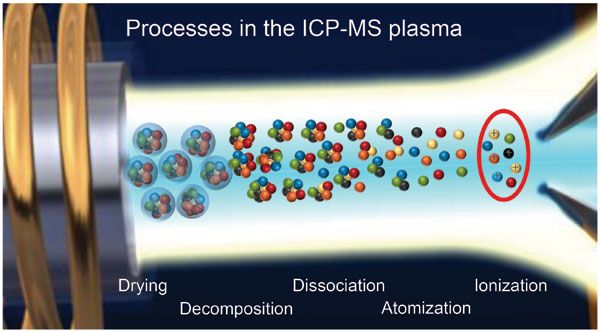
The most important factor in determining the accuracy of your Inductively Coupled Plasma Mass Spectrometry measurements is the sample volume. The larger the sample volume relative to the mass transfer velocity, the more accurate your measurement will be. Volume errors can be caused by many factors including too high vapor pressure of liquids or gases, or too low vapor pressure due to excessive liquid flow rate during processing. Another source of error may arise if a substance contains ions that have different masses but have similar chemistries.
In this case, standardizing on one ion may not provide accurate results because both will react with each other differently. There are many companies that provide this type of machine and one of them is the Agilent ICP MS instrument, and if you are having trouble optimizing your ICP MS measurements, read on!
Sample and gas flow rate set up
To measure the amount of analyte in a sample, you need to know what percent of the total volume is needed for your measurement. For example, if you want to analyze 5 uL from a given analyte solution, then you need 5 uL worth of solution for each experiment run (unless there are other factors affecting how much should be used).
This can be calculated by dividing the total volume of all samples by their mass concentration; in this case, that would be 100 mg/mL (milligrams per milliliter). The resulting number represents how many moles/grams or milliliters must be measured over time until reaching theoretical endpoint concentrations (e.g., 0).
The liquid sample introduction device
A liquid sample introduction device (LSID) is a fast and convenient way to introduce samples into the sample cell. The LSID should be able to deliver a stable flow rate that will maintain this flow throughout your analysis, even if you need to change instruments at different stages in your experiment.
It should also have an accurate metering system so that you can accurately measure each of your samples for analysis by ICP-MS or other methods such as atomic absorption spectroscopy (AAS).
Plasma conditions
The plasma is the ion source of your instrument, and it’s essential that you know how to control its conditions. Surprisingly, these are not very complicated; they’re simply a few parameters in your RF power supply (RF power) and gas flow rate.
Performance check
Once you’re done optimizing your ICP-MS, it’s time to check the instrument performance. This means checking the instrument response and stability, as well as its repeatability and accuracy.
- Instrument Response Time: This is how fast a measurement can be performed on the instrument. It is often expressed in seconds or picoseconds (ps).
- Repeatability: Repeatability refers to the repeatability within a certain range of measurements (typically ±0.2%).
For example, if one sample has an average value of 250 mg/L and another has an average value of 250 μm/L then both have excellent repeatability because their differences are less than 0.2%.
Choice of instrument mode
The instrument mode you choose is important because it determines the analytical precision, sensitivity, and accuracy of your measurements. In general, there are three main types of instrument modes:
- Auto-Pulse (AP) – this mode uses a fixed pulse repetition rate that can be adjusted by selecting one of several frequencies. AP is typically used to measure compounds at low concentrations in dilute solutions that are stable with repeated sampling over time periods ranging from seconds to hours or even days.
These conditions are often encountered when working with organic liquids such as ethers or esters; however, AP also works well for some inorganic compounds such as barium hydroxide solutions used for calibration purposes since these materials have similar properties to organic samples (i..e., they have low ionization constants).
- Standard-dilution Interference Detection (SDID) – SDID uses a single scan measurement cycle where each sample point is individually detected on a separate detector element within its own column within an area called “detector plate” which contains multiple rows of detectors arranged vertically along its length – much like a cathode ray tube TV screen! Each row has eight columns per side so there’s enough room left over after all rows have been filled up before starting another run so no need here either.
Signal processing method
The signal processing method used depends on the type of measurement you are performing. For example, if you have an ICP-MS sample with a high mass concentration and low molecular weight ( 30 kDa), then a collision/reaction interface (C/RI) might be necessary to optimize data acquisition conditions during the analysis process.
Optimize the collision/reaction interface (C/RI) between the source and the detector (quad) chamber
The C/RI is the interface between the source and the detector (quad) chamber. It consists of four regions:
- Reaction interface, where compounds form during collision or reaction with analytes. This area is defined by an aluminum foil with holes drilled in it, which allows for sampling of this layer by using an array of channels at various depths.
- Source chamber, where gases pass through before being sent to the quad (detector) chamber. This area can also be used as a reference point when measuring other properties such as gas pressure or temperature at different points along its length (for example, near its ends).
- Detector chambers contain detectors that measure light intensity levels that correspond directly with absorption spectrum peaks found in each element’s transition region. These chambers are often referred to as “quad” chambers because they contain four detectors separated vertically from each other by aluminum foils.
Quad chambers allow us access to some very interesting areas within the system like light-induced electron transfer processes taking place inside these elements!
Conclusion
We hope this guide has been helpful in helping you get started with your own ICP MS instrument measurements. We recommend that you start by using the basic methods outlined above, which will give you an idea of how to optimize your analysis results.